Highlights
-
•
Li-ion battery recycling is a mitigation measure for the supply risk of some critical raw materials
-
•
Hydrometallurgical recycling is potentially more sustainable than other methods
-
•
Highly efficient separations employing ILs and DESs as receiving phases can be realized on the lab scale
-
•
ILs & DESs present advantages over conventional solvents, their cost remains the main limit to reach industrial-scale processes
-
•
Supported liquid membranes can help to overcome someILs and DESs drawbacks
Abstract
The widespread adoption of lithium-ion batteries (LIBs) in modern electric vehicles has successfully addressed the issues of limited oil and gas resources, as well as environmental degradation. This development is crucial for achieving “carbon neutrality” and reduce “carbon peaking”. Proper disposal of used LIBs is vital for effective resource management and avoiding pollution and potential hazards associated with toxic substances. Moreover, a significant weight fraction of LIBs is constituted by so-called critical raw materials (CRMs), presenting a high supply risk and price variability. Over the past few years, considerable progress has been made in developing processes for the safe treatment and material recovery from spent LIBs, besides the recovery of CRMs. Among them, hydrometallurgical recycling of LIBs components employing affordable and eco-friendly ionic liquids (ILs) and deep eutectic solvents (DESs) has gained significant attention for their potentially superior selectivity, low energy consumption, and environmental impact. Such liquids present various advantages over traditional organic solvents employed in liquid separations in terms of volatility, safety, chemical and thermal stability, recyclability, and selectivity. This review provides an overview of the most recent advances in the application of ILs and DESs to recover CRMs from LIBs.
Graphical Abstract
Abbreviations
1-Butyl-3-methylimidazolium bistrifluoromethylsulfonylimide
1-Butyl-3-methylimidazolium tetrafluoroborate
1-Carboxymethyl-3-methylimidazolium bistrifluoromethylsulfonylimide
1-Ethanol-3-methylimidazolium bistrifluoromethylsulfonylimide
1-Ethyl-3-methylimidazolium chloride
2-Ethylhexyl phosphonic acid mono-2-ethylhexyl ester
2-Hydroxy-5-nonylaceto-phenone oxime
3-Methyl-1-octylimidazolium thenoyltrifluoroacetone
3,4,5-Trihydroxybenzoic acid
Carboxymethyl trimethyl bistrifluoromethylsulfonylimide
Di2-ethylhexylphosphoric acid
Dimethylbetapropiothetin
Guanidine hydrochloride
Methyltrioctylammonium lineolate
Methyltrioctylammonium oleate
N,N,N′,N′-Tetraoctyl diglycolamide
Pentapotassium bisperoxymonosulphate bissulphate
Polyethylene glycol
Polymer inclusion membrane
Polyvinylidene fluoride
p-Toluene sulfonic acid
Supported ionic liquid membrane
Supported liquid membrane
Tetrabutylammonium bisulfate
Tetrabutylammonium chloride
Tetrabutylammonium thiocyanate
Tetraethylammonium chloride
Trichloroisocyanuric acid
Trihexyltetradecylphosphonium thiocyanate
Trihexyltetradecylphosphonium bis2,4,4-trimethylpentylphosphinate
Trihexyltetradecylphosphonium bromide
Trihexyltetradecylphosphonium chloride
Trioctylmethylammonium chloride
Trioctylmethylammonium thiocyanate
Trioctylphosphine oxide
Volatile organic compound
Keywords
hydrometallurgy
Li-ion batteries
critical raw materials
ionic liquids
deep eutectic solvents
1. Introduction
Electric vehicles (EVs) have become the primary choice for countries worldwide to tackle the urgent problem of the climate change caused using fossil fuels since the first industrial revolution. The non-polluting and zero-emission nature of EVs has contributed to their exponential growth in global sales over the past decade, with approximately 5.1 million EVs on the road in 2018 [1]. This rapid development of EVs has led to a significant increase in the demand for batteries [2]. Specifically, lithium-ion batteries (LIBs) are used for energy storage in EVs, as well as in numerous consumer goods due to their long life cycle, high energy density, small self-discharge effect, high working voltage, no memory effect, wide applicable temperature range, and green environmental protection [3], [4].
The supply chain of the materials needed for manufacturing LIBs could face serious issues as the production is projected to range between 0.33 and 4 million tons per year from 2015 to 2040 [5]. In parallel, by 2030 the demand for Li and Co is estimated to increase by eighteen and five times, respectively, compared to current supply levels [6]. The expected rise in demand, combined with the limited number of suppliers (vide infra), characterizes multiple constituents of LIBs (Co, Li, P, Cu, graphite) as critical raw materials (CRMs) [7].
Several alternatives aim to replace LIBs to avoid CRMs like Co or Li. Sodium ion batteries are a promising alternative due to the abundance of Na in nature. However, the theoretical capacity of Na batteries is lower compared to LIBs [8], [9], [10]. Potassium ions are expected to have an even smaller desolvation energy than Li- and Na-based systems in aprotic solvents but could be easier trapped in the structure of the anode compared to smaller Na+ and Li+. Furthermore, the large ionic radius makes the preparation of oxides with high stoichiometry of K for cathode materials difficult [11]. Multivalent ions like Mg2+, Ca2+, Zn2+, or Al3+ can increase capacity, but practical implementation of electrolytes and cathodes remain limited to research laboratories. The main challenge lies in the low gravimetric energy densities, typically in the order of a few hundred Wh/kg, coupled with limited demonstrated durability and slow kinetics [12]. Aqueous zinc batteries offer unique properties, but face issues like limited potential window, electrode material dissolution and dendrite formation [13]. Al has the highest volumetric and good gravimetric capacity, is stable in the air atmosphere and is the most abundant element in Earth’s crust. However, Al has less negative standard reduction potential than the other metals, leading to a lower energy density. Moreover, there are challenges related to lifecycle, corrosion, refueling, efficiency, which limit their widespread adoption and make them better suited for specific niche applications where their advantages outweigh their disadvantages [14]. Cobalt-free cathodes like lithium iron phosphate offer cost and sustainability advantages, but may have lower energy density [15].
Remanufacturing and repurposing of used battery packs require partial disassembly, processing, testing and repacking of the battery cells are considered important stages of the value chain (Fig. 1), but not sufficient to mitigate the dependence on CRM to address the market demand [16].
 Fig. 1
Fig. 1The main strategy to relieve the pressure on the supply chain is to develop economically competitive recycling processes characterized by high recovery rates, low energy, and resources. Future supply crisis can only be prevented through 100 % LIB recycling with at least 90 % metal recovery or through the technologically, economically, environmentally friendly, and efficient metal recovery from low-grade primary resources [17]. Despite exploratory mining efforts, the global suppliers of CRMs employed in LIBs manufacturing (Co, Li, graphite, etc.) are limited to some countries [18]: for example, in 2022 Democratic Republic of Congo produced about 68 % of the total Co worldwide [19]. Numerous LIBs component materials including Co, Li, phosphorus, and graphite (Table 1), remain on the 2023 CRMs list [20]. With an estimated 21 million end-of-life (EoL) LIB packs expected to be generated between 2015 and 2040 [21], the recycling of spent LIBs becomes crucial. Notably, in 2019, China managed to recover over 36 % of spent LIBs in terms of quantity [22], whereas in 2022, the United States lagged significantly behind, with less than 1 % of spent LIBs being recycled [23].
| Components | Materials | Weight percentage (%) |
|---|---|---|
| Anode | Graphite | 15–20 |
| Cathode | LCO, LMO, LPF, NMC, LNMO | 25–30 |
| Binder | PVDF | 3 |
| Electrolyte | LiPF6, LiBF4, LiAsF6, LiClO4, PC, EC, DMC | 10 |
| Separator | PE, PP | 1–2 |
| Current collectors | Al, Cu | 10–20 |
This need for sustainable recycling processes promoted a vast number of fundamental and applied studies for CRMs recovery from EoL LIBs: a search conducted on the Web of Science database on May 2, 2024, using the terms “LIBs” and “recycling” revealed a growing interest within the scientific community on the topic (Fig. 2).
 Fig. 2
Fig. 22. Challenges in LIB recycling
Cathodes are key components of LIBs which determine the performance, and their composition is often closely linked to the specific application. Additionally, cathodes contain most weight fraction of the CRMs which could be potentially recovered. Metal oxides are commonly used as cathode materials in LIBs (Table 2), each with different performance, while graphite is the most common material utilized as anode. Among the cathodes reported in Table 2, NMC are currently dominating the market, mostly due to their high energy density and, according to a recent paper, are expected to reach 80% of the total storage capacity by 2025 [27].
| Cathode types | LCO | LFP | LMO | NCA | NMC | LNMO |
|---|---|---|---|---|---|---|
| Full name | Lithium Cobalt Oxide | Lithium Iron Phosphate | Lithium Manganese Oxide | Lithium Nickel Cobalt Aluminum Oxide | Lithium Nickel Manganese Cobalt Oxide | Lithium Nickel Manganese Oxide |
| Chemical formula | LiCoO2 | LiFePO4 | LiMn2O4 | Li(Ni,Co,Al)O2 |
LiNi0.33Mn0.33Co0.33O2 (NMC111) |
LiNi0.5Mn1.5O4 |
|
LiNi0.5Mn0.3Co0.2O2 (NMC532) |
||||||
|
LiNi0.6Mn0.2Co0.2O2 (NMC622) |
||||||
|
LiNi0.8Mn0.1Co0.1O2 (NMC811) |
||||||
| Crystal structure | Layered | Olivine | Spinel | Layered | Layered | Spinel |
| Safety | Moderate | Excellent | Very good | Good | Good | Excellent |
| Energy density | Very good | Good | Good | Excellent | Excellent | Excellent |
| Power density | Good | Very good | Very good | Very good | Good | Very good |
| Cycle lifespan | Good | Very good | Good | Very good | Good | Very good |
| Performance | Very good | Very good | Good | Very good | Very good | Very good |
| Cost | Poor | Very good | Very good | Very good | Good | Very good |
| Market | Outdated | Electric bikes, buses | Small | Steady | Growing (from NMC111 to no-Co chemistries) | Electric vehicles |
A first reason to recycle the LIBs is to prevent the hazard related to the highly toxic materials contained. As an example, the LIBs cathodes, typically contain high concentrations of Co and Ni (Table 3) which are known to pose significant risks to living organisms [31] if dispersed in the environment in an uncontrolled manner. Furthermore, other non-metallic components, such as fluorine-containing materials (e.g. hexafluorophosphates in electrolyte and the PVDF binder) may generate fluorine pollution of soil and water [32].
| Material | NMC111* | NMC532* | NMC622* | NMC811* | 2022** | 2020** |
|---|---|---|---|---|---|---|
| (kg) | (USD) | |||||
| Lithium | 0.141 | 0.136 | 0.118 | 0.1 | 27,327 | 7250 |
| Nickel | 0.351 | 0.508 | 0.531 | 0.6 | 32,424 | 15,090 |
| Cobalt | 0.352 | 0.204 | 0.178 | 0.75 | 81,860 | 33,000 |
| Manganese | 0.328 | 0.285 | 0.166 | 0.07 | 2400 | 1565 |
| Aluminum | 3.11 | 3.07 | 3.017 | 2.921 | 3048 | 1582 |
| Copper | 0.677 | 0.661 | 0.605 | 0.549 | 9820 | 6788 |
| Graphite | 0.978 | 0.981 | 0.96 | 0.961 | 3800 | 3400 |
* “BatPaC,” 2020; ** “Benchmark Minerals, Lithium carbonate prices break through 40USD/kg barrier,” 2022; “Fastmarkets: Graphite pricing,” 2022; “Futures price of cobalt worldwide from August 2019 to August 2023,” 2023; “Markets Insider, Business Insider Commodities: Aluminum price,” 2022; “Markets Insider, Business insider commodities: copper price,” 2022; “Markets Insider, Business Insider Commodities: nickel price,” 2022; “SMM, Price metal-Manganese,” 2022.
Also, a practical reason supports the recycling of LIBs: spent batteries contain valuable metals at much higher concentrations than those found in industrial ores [33]. Thus, this process of “secondary mining” would be much more efficient than processing natural minerals. For instance, the Li content of spent LIBs is approximately 10 times that of primary Li ore (lithium pyroxene) and 30 times that of Li carbonate salts found in brine pools [34]. Additionally, the escalating prices in metal supply chains, particularly for NMC cathode components (Table 3), further highlight the pressing need for LIB recycling.
3. Battery preparation for recycling
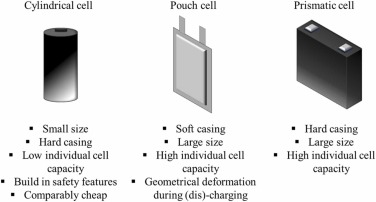
The recycling process involves sorting, discharging, and dismantling, with one major challenge being the diverse assembly of cells, modules, and packs. Manufacturers utilize various cell geometries, such as prismatic, cylindrical, and pouch cells (Fig. 3), making disassembly difficult [35]. Battery cells are often tightly sealed and assembled in modules and packs using different adhesives. Active electrode materials, in granular form, are attached to collector plates using binders like PVDF, known for its resistance to high temperatures and non-reactivity with battery components [36]. Efficient cell dismantling and recycling face hurdles primarily due to the intricate assembly of packs and modules.
 Fig. 3
Fig. 33.1. Mechanical processing
The discharging process is often done by immersion in a concentrated saline solution. This step is required to reduce the risk of combustion, explosion, and toxic gas emissions for high voltage cells.
For EoL battery recycling, wet and dry crushing methods are used: wet crushing involves using a water or salt solution to create a cool, oxygen-free environment, but generates wastewater and may over-crush foils, while dry crushing produces fewer impurities [37], [38]. Sorting, sieving, and magnetic separation are then employed to separate fragments. Pre-treatment methods like ultrasonic [39], microwave [40], solvent [41], [42], or thermal treatment [43], [44], [45] were used before for separating active electrode material from current collector foil. Mechanical processes like milling or sieving may also be necessary to disrupt crystal structures [46], [47], [48].
3.2. Binder removal
In LIBs, a binder serves as a crucial component within the electrode structure, facilitating the adhesion of active material to the current collector. Typically composed of polymers (PVDF, PAA, PVA), binders ensure mechanical integrity and promote stable electrochemical performance by securely holding together the electrode's active materials during charge and discharge cycles. Binder removal from cathode material poses a significant challenge in battery recycling. High temperature pyrolysis is effective, but energy-intensive and environmentally impacting, due to toxic gas emissions [21], [49], [50]. A more efficient method is dissolution of binder utilizing proper solvent. Common processes for PVDF binder removal are shown in Table 4, Table 5.
| Method | Temperature (°C) | Time | Efficiency (%) | Ref. |
|---|---|---|---|---|
| Thermal processes | ||||
| Pyrolysis with CaO | 300 | 10 min | 97 | [49] |
| Pyrolysis under air | 150–500 | 1 h | 100 | [21] |
| Pyrolysis under nitrogen | 550 | 3 h | 100 | [50] |
| Dissolution processes | ||||
| Diacetone alcohol | 165 | 2 h | 8.6 | [55] |
| Diethyl glycol monoethylene ether | 200 | 2 h | 10.7 | [55] |
| Acetone | 56 | 2 h | 12.3 | [55] |
| DMAc | 160 | 2 h | 31.4 | [55] |
| DMF | 150 | 2 h | 33.1 | [55] |
| 5 M NaOH + catalyst | 80 | 2 h | 60.5 | [55] |
| ChCl:glycerol (2.3:1) | 190 | 15 min | 100 | [56] |
| Citrus juice | 90 | 20 min | 100 | [57] |
| N-Methyl-2-pyrrolidone | 100 | 1 h | 100 | [58] |
| DMSO | 60 | 85 min | 100 | [59] |
| 5 M KOH + catalyst | 80 | 2 h | 100 | [55] |
| ChCl:EG nanofluids | 100 | 10 h | 100 | [60] |
| Recycling method | Pros | Cons |
|---|---|---|
| Pyrometallurgy | Process simplicity; high productivity; large scale application; commercially viable, high efficiency. | Gas clean-up is required to avoid the release of toxic emissions; high energy consumption; material loss (Li in the slag); additional process needed for metal separation, high energy consumption. |
| Direct recycling | Can recover all battery components; is economically viable for cheaper battery chemistries like LFP and LMO; no strong acid consumption; short recovery route. | Requires single cathode input; degradation may limit repetition; not demonstrated at larger scale; high operational and equipment requirements; incomplete recovery. |
| Hydrometallurgy | Low cost; low energy consumption; process flexibility; high recovery efficiency; high purity of products; no gaseous emissions. | Big dependence on pre-treatment; acid breaks down original cathode structure; large amount of VOC; only economical for batteries containing Co and Ni; wastewater productions. |
Mechanical processes offer a low-cost, simple, and flexible alternative for binder removal and carbon black separation from cathode composites. Common methods include grinding, sieving, magnetic, electrostatic or pneumatic separation, and flotation [51]. Delamination involves crushing material into layers [44] utilized vibrating screens to separate black mass and graphite from Al and Cu foil, while [52] used high-powered ultrasound for rapid delamination. Also ionic liquids (ILs), like [C4mim][BF4], dissolve PVDF at lower temperatures with high efficiency [53].
Spouted bed elutriation is another method that implements separation of particles based on their size, shape and density, utilizing a stream of gas or liquid flowing in an opposite direction of sedimentation [54].
4. Methods of recycling
After pretreatment, electrode materials are collected in an intermediate product, the so-called “black mass”, which contains all valuable metals such as Co, Mn, Ni, and Li [61] and is then subjected to the separation and purification stages [62]. Metal recovery can be achieved through a range of technologies. The metallic fraction undergoes processes like hydrometallurgy [63], [64], pyrometallurgy [65], [66], combination methods [67], [68], [69], and direct recycling [70], [71] as depicted in Fig. 4.
 Fig. 4
Fig. 44.1. Pyrometallurgy
Pyrometallurgical technology is commonly used in the industrial recycling of LIBs as it employs thermal treatment to reduce metal oxides into an alloy. This process is advantageous due to its high processing capacity [16], simple operation, no need of chemical pretreatment. The primary environmental concerns associated with pyrometallurgy are the high-energy consumption and significant gaseous emissions. Furthermore, the process is unable to recover all battery components, as organic compounds are converted to pure carbon. The alloy produced by this process is mixture of different metals present in the LIBs and requires additional processing, to recover pure metals [72].
4.2. Direct recycling
Cathode materials can undergo regeneration through direct recovery pathways, such as restoring the crystal structure while simultaneously compensating for the loss of Li electroactivity in situ. This approach is motivated by the fact that the decline in battery performance often stems from an insufficient supply of active Li and irreversible structural transitions [73], [74]. In contrast, direct recycling is a low-cost alternative that retains the cathode structure of LIBs instead of dissolving, extracting, and reassembling materials. This method establishes its cost-effectiveness in relation to other methods by minimizing intervention on the active material [75]. It has been argued that full repurposing of battery requires a comparable number of steps as the leaching/re-synthesis process, potentially negating any economic advantage. Wang et al. [76]introduced an innovative ionothermal approach for the regeneration of NMC111 using ILs, specifically [C2OHmim][Tf2N]. Unlike conventional solid-state and hydrothermal methods, the ionothermal method offers the advantage of regenerating cathode active materials at relatively low temperatures (150–250 °C) and under ambient pressure. Notably, the combination of [C2OHmim][Tf2N] and LiCl at 150 °C yielded the highest discharge capacity retention (approximately 99 %) among the various regeneration techniques employed for NMC111. Shi et al. [73], demonstrated re-lithiation with LiNO3:LiOH (3:2) deep eutectic solvent (DES) at 300 °C. Li content can be fully restored after 4 h. A short annealing step after re-lithiation is necessary so NMC523 particles can be completely restored to their original pristine composition. Without annealing step, cathode contains many amorphous regions which can decrease battery electrochemical performance.
Establishing and maintaining a direct recycling system can be expensive, especially when specialized equipment and processes are required for each battery type. The costs associated with collection, sorting, and processing may not always be economically viable [70].
4.3. Hydrometallurgy
Hydrometallurgical processes have the advantage of producing exceptionally pure products from low-grade ores and being low energy-intensive [41]. Traditional hydrometallurgical processes employ volatile organic compounds (VOCs) or multiple extracting ligands to extract valuable metals and separate different metals present in the leachates [77]. Although VOCs can be separated from the reaction medium, it can pose serious threats to living organisms. Exposure to VOCs through skin contact, inhalation, or ingestion can cause irritation, nausea, and dizziness, and long-term exposure may lead to liver, kidney, and central nervous system damage [78]. Additionally, VOCs present serious safety issues as they are highly flammable.
The pursuit of sustainable hydrometallurgical processes for LIBs processing using safer solvents has been subject of numerous studies in the last two decades.
Compared to conventional VOCs, ILs and DESs display high thermal and chemical stability, a wide electrochemical window, negligible vapor pressure and lower toxicity [81], [82]. Additionally, DESs offer benefits like easier preparation methods, enhanced purity of synthetic products, reduced costs, less corrosive impact on equipment, relatively straightforward biodegradation, and generally lower toxicity [82].
This review focuses on the recent advancements in the hydrometallurgical recovery of CRMs from LIBs using ILs and DESs, as depicted in Fig. 5. It is organized as follows: i) introduction of ILs and DESs and properties relevant to separation processes; ii) leaching and recovery of the CRMs by liquid-liquid extractions employing ILs and DES as receiving phases; iii) application of ILs/DES in supported liquid membranes; iv) status of industrial applications of separations employing ILs/DES; v) conclusions and outlook.
 Fig. 5
Fig. 55. ILs and DESs for hydrometallurgical applications
5.1. Ionic Liquids
ILs are salts formed by an organic cation and an organic or inorganic anion which are in the liquid state at temperatures below 100 °C [83]. The structure of some common anions and cations, for LIBs recycling, are shown in Fig. 6. When in the liquid state at room temperature, they are also called Room Temperature ILs (RTILs) [84].
 Fig. 6
Fig. 6Understanding the structure and thermodynamic characteristics of metal ion solvation within specific solvents [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95] and ILs [96], [97], [98], [99], [100], [101], [102] is crucial for a precise understanding of chemical phenomena within the liquid. This understanding is not only fundamental but also pivotal for practical applications [102], [103], [104], [105], [106]. The evolution in IL composition over the years demonstrates a growing sophistication in tailoring their properties to meet specific application requirements [107], [108], [109].
The physical, chemical, and thermal properties of ILs are significantly influenced by the selection of cations, including their nature, length, and symmetry, as well as the choice of anions, considering their structure and charge distribution [110], [111], [112]. For instance, variations in the anion composition within the IL structure can strongly impact its melting point, viscosity, thermal properties, and hydrophobicity [113], [114].
In hydrometallurgical solvent extraction processes, modifications in the composition of ILs can result in a variety of behaviors in metal interactions, thereby influencing the efficiency and mechanism of extraction [115], [116]. Depending on the extraction conditions, such as the composition of the aqueous feed solutions, and the presence of auxiliary extracting molecules in the IL phase, different mechanisms can be identified [117], [118]:
- Neutral extraction: This mechanism involves the formation and extraction of a neutral complex in the IL phase. While it shares similarities with conventional solvents, the detailed mechanisms can vary. In this mode, the IL functions as a polar non-aqueous solvent [119].
- Cation exchange: Unique to IL systems, this mode entails the IL acting as a liquid ion exchanger, transferring cationic complexes from the aqueous phase into the IL phase. Consequently, an IL cation shifts to the aqueous phase while a metal cation moves to the IL phase to maintain electro-neutrality [120], [121].
- Anion exchange: Also specific to IL systems, this mode involves the formation of over-neutralized anionic complexes that can be transferred to the IL phase. In exchange, an IL anion moves to the aqueous phase. It's worth noting that anion exchange is less common compared to cation exchange [122], [123].
The ion-exchange mechanisms pose a significant drawback in IL-based extraction systems, as a portion of the IL is lost into the aqueous phase, essentially acting as a sacrificial component. The primary factor influencing this exchange mechanism is the solubility of the IL component in the aqueous phase [124]. Furthermore, increasing the hydrophobicity, such as by extending the alkyl chain of the IL cation, can shift the mechanism from ion exchange to neutral complex extraction [125], [126].
5.2. Deep Eutectic Solvents
DESs were introduced as a family of solvents derived from ILs in the early 2000s by Abbott et al. [127], who described eutectic mixtures whose melting point is much lower than those of the pure components (Fig. 7). They are formed by a compound that acts as hydrogen bond acceptor (HBA), typically a quaternary ammonium salt, and either a metal salt or an organic compound that acts as hydrogen bond donor (HBD) [128].
 Fig. 7
Fig. 7Common HBAs and HBDs employed in the preparation of DESs are inexpensive starting materials, some of which are shown in Fig. 8 [129]. The latter features make DESs interesting in terms of economic sustainability and scaling of the process [130].
 Fig. 8
Fig. 8The properties of DESs, such as conductivity, density, melting point, polarity, and viscosity, are influenced by their known composition. By adjusting the HBA:HBD ratio, one can tailor these properties to achieve different properties and characteristics [131], [132]. DESs offer advantages similar to ILs, but they tend to exhibit higher viscosities, posing a limitation in separative applications [133].
Although the viscosity problem can be solved quickly and easily in the case of DESs by varying the temperature or by using water as an additional component, a large excess of water can, in some cases, lead to the complete decomposition of the DES [134], [135]. Some properties of DESs used in the recycling LIBs are reported in Table 6.
| DES | Molar ratio | Melting point (°C) | Density (g cm–3) | Viscosity (cP) | Ref. |
|---|---|---|---|---|---|
| ChCl:EG | 1:2 | –66 | 1.12 | 36 | [136], [137], [138] |
| ChCl:glycerol | 1:2 | –40 | 1.18 | 376 | [136], [137] |
| ChCl:OXA | 1:1 | 34 | 1.24 | 89 | [136], [139], [140] |
| ChCl:PPA | 1:1 | 20 | - | - | [139] |
| ChCl:MAC | 1:1 | - | 1.24 | 541.1 | [141] |
| ChCl:PTSA | 1:1 | - | 1.19 | 170 | [142] |
| D2EHPA:TOPO | 1:1 | –100.1 | 0.94 | 88 | [143] |
| D2EHPA:menthol | 1:1 | - | 0.94 | 31.2 | [144], [145] |
| [A336][Cl]:menthol | 6:4 | −20.8 | 0.88 | 450.7 | [146] |
| [A336][Cl]:menthol | 3:7 | −9.9 | 0.89 | 257.6 | [146] |
| TOPO:decA | 1:1 | - | 0.88 | 39 | [147], [148] |
DESs can solubilize metal salts and oxides [149], making it possible to use them for the direct leaching of metals from primary or secondary resources to replace conventional leaching methods with acids [150], [151]. In Table 7 the distinctive properties of ILs, DESs, and VOCs are compared [152].
| VOCs | ILs | DESs | |
|---|---|---|---|
| Formed by | Complex process | Organic cation & organic/inorganic anion | Hydrogen bond donor & acceptor |
| Number of solvents | > 1000 | > 1000,000 | > 1000,000 |
| Melting point | < 0 °C | < 100 °C | < 100 °C |
| Preparation | Hard | Hard | Easy |
| Tunability | No | Yes | Yes |
| Viscosity | No | High [153] | High [154] |
| Thermolability | Labile | Stable up to 300 °C [155] | Stable up to 200 °C [156] |
| Volatility | High | Low [157] | Low [158] |
| Flammability | Flammable | Non-flammable [159] | Non-flammable [160] |
| Biodegradability | Challenging for multiple VOCs [161] | No [162] | Yes [163] |
| Recyclability | Low, for selective solvents | Yes [164] | Yes [165] |
| Cost | Low | High | Medium |
| Toxicity | High, many carcinogenic [166] | High to Aquatic biota [167] | Low [168] |
6. Recycling of metals from spent LIBs
Cathodes take up around 30 % of LIBs weight (Table 1). The conductor plate to which cathode is attached is typically made of Al, while the cathode material can contain various other metals which are economically viable for recycling (Table 2, Table 3) [36]. Because of this variety in composition, recycling is particularly challenging. Choosing a recycling method depends on the specific metal that needs to be recovered, as each method has varying recovery efficiencies that make them more suitable for certain types of battery materials [169].
6.1. Leaching
Leaching involves the dissolution of the desired material into a liquid solution, usually by treating the starting material, traditionally using a strong acid or base. In the case of leaching processes of waste cathodes from LIBs, essentially all protocols involve the use of mineral acids such HCl [58], [170], [171], [172], [173], HNO3 [174], [175], [176], [177] or H2SO4 [178], [179], [180], [181], [182], [183], [184], [185] at various concentrations (Table 8). Alternatively, weak acids which can be derived from biomass (citric [186], [187], [188], malic [41], [186], [187], succinic [189], etc.) have been tested, sometimes in combination with H2O2 [41], [175], [178], [179], [185], [186], [189], [190], [191]. The latter methods have been shown to be effective but are usually slower and need higher operating temperatures (Table 8). Besides the ability to dissolve the starting material quickly and efficiently, the leaching agents have a strong influence on the speciation of the metals in solution. For example, Co2+ ion is complexed with Cl− (e.g. CoCl42−) in concentrated chloride-containing solutions, while this is not the case when HNO3 is employed (Co2+ is present as aqua-ion) [192]. Therefore, this step determines the metal species which is extracted in the receiving phase. This is also true when weak carboxylic acids are used, as they can act as complexing agents for cations [193].
| Cathode type | Leaching media | Temperature (°C) | Time | Solid/Liquid ratio (g/L) | Leaching efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| LCO | 1 M HNO3 + 1 % H2O2 | 80 | 1 h | 20 | Li 100, Co 100 | [175] |
| 4 M HCl | 80 | 1 h | 100 | Li 100, Co 100 | [58] | |
| 0.5 M H2SO4 + 30 % H2O2 | 80 | 2 h | 18.52 | Li 100, Co 100 | [178] | |
| 2 M H2SO4 + 5 % H2O2 | 75 | 30 min | 100 | Li 94, Co 93 | [179] | |
| 3 M H2SO4 | 70 | 6 h | 200 | Li 98, Co 98 | [180] | |
| 1 M H2SO4 + 0.2 M NaCl + 30 % Cu powder | - | 2 h | 17.125 | Li 90, Co 90 | [181] | |
| 2 M H2SO4 + 5 mL of glucose after 2 h | 80 | 8 h | 74 | Li 92, Co 88 | [182] | |
| 1.5 M MAC + 2 % H2O2 | 90 | 45 min | 20 | Li 100, Co 90 | [41] | |
| 1.5 M MAC + 2 % H2O2 | - | 40 min | 20 | Li 100, Co 90 | [186] | |
| 1.5 M SAC + 4 % H2O2 | 70 | 40 min | 15 | Li 96, Co 100 | [189] | |
| 1.25 M CIT + 1 % H2O2 | 90 | 30 min | 20 | Li 100, Co 90 | [186] | |
| 1.5 M Aspartic acid + 4 % H2O2 | - | 2 h | 10 | Li 60, Co 60 | [186] | |
| 2 M Serine | 70 | 3 h | 10 | Li 80, Co 98 | [198] | |
| 1 % A. Niger | 30 | 40 days | 25 | Li 100, Co 82 | [195] | |
| LMO | 2 M HNO3 | 80 | 2 h | Whole battery cathode | Li 100, Mn 95 | [174] |
| NCA | 4 M HCl | 90 | 18 h | 50 | Li 100, Co 100, Ni 100, Al 100 | [170] |
| NMC | 1 M HNO3 | 25 | 2 h | 3 | Li 77, Mn 100, Co 91, Ni 99 | [176] |
| 1.75 M HCl | 50 | 24 h | 200 | Li 99, Mn 99, Co 99 | [171] | |
| 2 M HCl | 60–80 | 1.5 h | 20 | Li 100, Co 100 | [172] | |
| 5 M HCl | 95 | 70 min | 10 | Li 97.59, Co 99.74 | [173] | |
| 10 M HNO3 | 87.8 | 10 min | 20 | Li 80.5 | [177] | |
| 2.5 M H2SO4 + Al/Cu | 60 | 6 h | 50 | Li 98.23, Mn 98.64, Co 98.37, Ni 95.45 | [183] | |
| 2.5 M H2SO4 | 90 | 2.5 h | - | Li 99, Mn 99, Co 99, Ni 99 | [184] | |
| 4 M H2SO4 + 10 % H2O2 | 85 | 2 h | 100 | Li 96, Co 95 | [185] | |
| Aqua regia | 60 | 48 h | 100 | Li 100, Mn 100, Co 100, Ni 100 | [187] | |
| 1.5 M CIT | 95 | 30 min | 100 | Li 97, Co 95, Ni 99 | [187] | |
| 1 M MAC | 95 | 30 min | 100 | Li 96, Co 98, Ni 99 | [187] | |
| 1.5 M CIT + ultrasound | 50 | 24 h | 20 | Li 82, Mn 95, Co 81, Ni 74 | [188] | |
| 2 M FAC + 6 % H2O2 | 60 | 2 h | 50 | Li 99.9 | [190] | |
| 1.5 M LAC + 0.5 % H2O2 | 70 | 20 min | 20 | Li 97.7, Mn 98.4, Co 98.9, Ni 98.2 | [191] | |
| 0.75 M FA + microwave | 50 | 30 min | 15 | Li 93, Mn 96, Co 99, Ni 96 | [40] | |
| 3 M (NH4)2SO4+ 0.75 M (NH4)2SO3 | - | - | 83 | Li 98, Mn 92, Co 81, Ni 98, | [199] | |
| 1 M NH3 + 0.5(NH4)2SO3 + 1 M (NH4)2CO3 | 80 | 1 h | 10 | Li negligible, Mn negligible, Co 94, Ni 37 | [200] | |
| 40 g/L Acidithiobacillus ferrooxidans + Acidithiobacillus thiooxidans + 36.7 g/L FeSO4, pH = 1.5 | 30 | - | 5 | Li 99.2, Co 50.4, Ni 89.4 | [194] |
Microorganisms, such as bacteria and fungi, have been considered in recent years for their ability to extract metals from ores through a process known as bioleaching [194], [195]. This environmentally friendly technique utilizes the metabolic activities of microorganisms to dissolve metals from mineral ores, making them more accessible for extraction. Bioleaching offers several advantages over traditional chemical leaching methods, including lower energy consumption, reduced environmental impact, and the ability to process low-grade ores economically [196], [197].
6.2. Metal extraction processes with ILs
Several families of hydrophobic ILs have been tested as receiving phases in liquid-liquid metal extractions (see 5.1). The [P66614][TMPP] IL was applied in the recycling process of the NMC cathode powder [201], for the selective extraction of Co over Ni. In order to enhance the separation, a chelating extractant such as LIX 84-IC was employed [202]. Notably, [P66614][TMPP] IL demonstrates the ability to extract the metals through adduct formation with the phosphinate compound, especially when the acid concentration is relatively low [201]. This differs from another member of the phosphonium-based IL family, [P66614][Cl], where the exchange occurs between the chloride ions of the IL molecules and the anionic chloridometalates (MClxn−) [203], [204], observed at high HCl aqueous concentrations.
The separation of metals in NMC cathodes followed a specific sequence: Mn was initially separated using a mixture of TODGA diluted in [C4mim][Tf2N] IL [115], while Co was separated by [P66614][Cl] IL in H2SO4 media. Wellens et al. [205] utilized [P66614][TMPP] combined with another immiscible IL, [C2mim][Cl], and observed a high selectivity for Co over Ni. In another study, [P66614][Cl] was used to selectively extract Mg from Li in a LMO cathode at pH 5.5, achieving a remarkable extraction efficiency of 99.6 % for Mg [206]. To compare anion impact on the extraction efficiency, Dhiman and Gupta utilized [P66614][Br] IL and H2SO4, HCl, and HNO3-based leachate solutions for the extraction of Co from exhausted LIBs taken from mobile phones [207]. The study revealed that the efficiency of Co extraction increased proportionally with the concentration of HCl (Table 9). In contrast, the extraction yield remained consistently low when using samples containing H2SO4 and HNO3.
| IL | Cathode type | A:O ratio | Conditions | % Co recovered | Ref. |
|---|---|---|---|---|---|
| [P66614][Cl] | Synthetic NMC | 1:1 | 30 min, 25 °C, 9 M H2SO4 | 92.8 | [115] |
| LCO | 10 min, 60 °C, 0.5 M HCl | 17.7 | [209] | ||
| 10 min, 60 °C, 4 M HCl | 97.8 | ||||
| [P66614][Br] | Mixed | 1:3 | 10 min, 25 °C, 5 M HCl | 99 | [207] |
| 2:1 | 10 min, 25 °C, 5 M HCl | 69.8 | |||
| [P66614][TMPP] | NMC | 1:1 | 10 min, 25 °C, pH= 2.0 | ∼20 | [201] |
| 10 min, 25 °C, pH= 4.0 | ∼38 | ||||
| 10 min, 25 °C, pH= 5.4 | ∼86 | ||||
| 5 min, 25 °C, pH= 2.5 | ∼34 | [202] | |||
| 5 min, 25 °C, pH= 6.0 | 91 | ||||
| 10 min, 25 °C, pH= 2.5 | 34 | [210] | |||
| 10 min, 25 °C, pH= 6.0 | 90 | ||||
| 10 min, 60 °C, pH= 5.0 | 96 | ||||
| [P66614][SCN] | LCO | 1:1 | 10 min, 30 °C, pH= 2.0 | 49.7 | [208] |
| 10 min, 30 °C, pH= 4.5 | ∼85 | ||||
| 10 min, 30 °C, pH= 5.5 | 95.7 |
Conversely, [P66614][SCN] IL was employed to separate Co from Li from concentrated H2SO4 solutions [208]. Co exhibited a remarkable capacity to preferentially form the tetra thiocyanate complex [Co(SCN)4]2− by interacting with the IL's anion. This interaction occurred through a split-anion extraction mechanism.
When the ammonium-based ILs, [A336][SCN] and [A336][Cl], were employed, the recovery of Co was 10.5 % with [A336][Cl], whereas the efficiency of Co extraction reached 94.3 % when undiluted [A336][SCN] was used under the condition of 10 min at 30 °C (Table 10) [211].
| IL | Cathode type | A:O ratio | Conditions | % Co recovered | Ref. |
|---|---|---|---|---|---|
| [A336][SCN] | LCO | 1:1 | 15 min, 30 °C | 94.3 | [211] |
| [A336][Cl] | 10.5 | ||||
| [N1888][Ol] | Synthetic | 1:1 | 15 min, 25 °C | >95–99 | [213] |
| [N1888][Linol] | |||||
| [Omim][TTA] | LCO | 3–5:1 | 120 min, RT | 88 | [217] |
Nevertheless, the widespread use of ILs is hindered by excessive costs, environmental issues, and recycling problems. Bio-based ILs offer a greener alternative, as they are obtained from renewable sources like amino acids, sugars, or fatty acids. Anions derived from fatty acids, such as oleate and linoleate, remain immiscible with water, and tertiary amines and quaternary ammonium salts demonstrate effective anion exchange. Moreover, the use of bulky, long-chained tetraalkylphosphonium cations and hydrophobic oleate anions helps to prevent the loss of the IL to the aqueous phase [212]. Two hydrophobic fatty acid-based ILs were synthesized, [N1888][Ol], and [N1888][Linol]. These ILs were used in a single-stage extraction process to extract Co and Ni from an aqueous solution, demonstrating high efficiency even at low concentrations [213].
Nevertheless, amino acids have good prospects in industrial applications because of their high biocompatibility, low price, and excellent availability. Five amino acids (glycine, phenylalanine, serine, glutamine, and glutamic acid) were investigated in [N4444][HSO4] IL on separation of Co and Ni from Mn [214].
Also ILs containing the [TTA]− anion of ILs, have also been studied in the field of metal extractions [215], [216]. The [Omim][TTA] IL used for the selective recovery of Co from a LCO cathode leached using TAC [217] (Table 10) gave a high extraction rate for Co 88 % with all Li retained in the aqueous phase.
A novel processes for the selective extraction of Li from spent NMC532 cathode by two carboxyl-functionalized ILs ([HO2C3MN][Tf2N] [218] and [HO2Cmim][Tf2N] [219], Table 11) using TBP as auxiliary extractant. Despite employing the same cation-exchange mechanism, the stoichiometry of extraction differs between the two ILs. In the former case [218], the extractive stoichiometry revealed the formation of a 3:1 complex between TBP molecules and Li ions. Conversely, when utilizing [HO2Cmim][Tf2N] in conjunction with TBP [219], a 1:1 complex between Li ions and TBP molecules was formed.
| IL | Cathode type | TBP:IL ratio | Conditions | % Li recovered | Ref. |
|---|---|---|---|---|---|
| [HO2C3MN][Tf2N] | NMC532 | 8:2 | 20 min, 25 °C, pH= 3.0 | 63.2 | [218] |
| 6:4 | 48.7 | ||||
| [HO2Cmim][Tf2N] | NMC532 | 8:2 | 30 min, 25 °C, pH= 1.0 | 31.2 | [219] |
| 30 min, 25 °C, pH= 3.0 | 82.7 | ||||
| 30 min, 25 °C, pH= 5.0 | 82.1 | ||||
| 6:4 | 30 min, 25 °C, pH= 5.0 | 71.3 |
In a recent development, researchers successfully utilized a [TBP]-based IL, denoted as [TBP][DTPA], in aqueous sulfate solutions for extracting Li and Co from a spent LCO battery [220].
6.3. Metal extraction processes with DESs
Several types of DES could be utilized for LCO cathode leaching. Acidic components of DESs [221], [222], [223], [224], additives [225], or microwave radiation [226] reduce leaching time or temperature, as depicted in Table 12. By adjusting the temperature and leaching time, solid liquid ratio doesn’t play a significant role in leaching. In all cases, Co and Li leached more than 90 %. Yan et al. [221] and Tian et al. [227] both utilized GH:LAC DES. Leaching time and solid:liquid ratio was the same, but because of AAC addition by Yan et al., leaching temperature decreased by 30 °C, while leaching efficiency decreased by only 2–3 %.
| DES | Temperature (°C) | Time | Solid/Liquid ratio | % recovered | Ref. |
|---|---|---|---|---|---|
| GH:LAC (1:2) + AAC | 50 | 24 h | 1:50 | Li 97.42, Co 96.91 | [221] |
| ChCl:PTSA·3 H2O | 90 | 15 min | 1:20 | Co 94 | [228] |
| PTSA:PolyEG (1:1) | 100 | 24 h | 1:50 | Co 99.5 | [229] |
| ChCl:PTSA | 150 | 72 h | 1:50 | Co 75.0 | [230] |
| ChCl:EG | 87.5 | 2 h | 1:50 | Li 100, Co 100 | [232] |
| ChCl:THBA | 110 | 12 h | 1:67 | Li 98.04, Co 98.04 | [233] |
| ChCl:OXA | 180 | 10 sec | 1:150 | Li 100, Co 100 | [222] |
| ChCl:OXA | 110 | 2.5 | 1.50 | Li 100, Co 100 | [223] |
| ChCl:FAC + 10 % H2O + microwave radiation | 70 | 10 min | 1:50 | Li 100, Co 100 | [226] |
| ChCl:LAC (1:2) | 105 | 5 h | 1:77 | Co 95, Li 95 | [224] |
| ChCl:MAC | 150 | 72 h | 1:50 | Li 98.78, Co 98.61 | [230] |
| ChCl:benzenesulfonic acid:ethanol (1:1:2) | 90 | 2 h | 1:50 | Li 99, Co 98 | [234] |
| ChCl:PTSA:MAC | 150 | 72 h | 1:50 | Li 98.78, Co 98.61 | [230] |
| ChCl:glycerol | 200 | 20 h | 1:50 | Co 95.7 | [235] |
| ChCl:CIT + H2O | 120 | 4 h | 1:50 | Li 100, Co 97.6 | [236] |
| BHC:LAC | 120 | 2.2 h | 1:50 | Li 99.98, Co 99.86 | [237] |
| EG:TAC | 120 | 12 h | 1:50 | Li 98.34 | [238] |
| DMT:OXA + H2O | 60 | 15 min | 1:40 | Li 99.8, Co 0.5 | [225] |
PTSA is an organic acid, and when used as a HBA in DES, it imparts acidity of the solvent mixture. While, ChCl is a quaternary ammonium salt and contains a positively charged nitrogen atom, making it a potential proton donor that also impacts acidity of DES. Hydrated PTSA was employed by Roldán-Ruiz et al.[228] and Chen et al. [229]. By utilizing DES with hydrated PTSA, leaching temperature is almost two times lower, while leaching time decrease 280 times compared to dry PTSA [230]. Moreover, efficiency increase from 75 % to 94 %. Compared to organic acids, PTSA:ChCl-based DESs offered a significant reduction of the solute-to-solvent ratio needed for full Co dissolution. This brings economic and sustainability benefits and promising scalability due to low solvent volumes necessary. Using PTSA:ChCl-based DESs for leaching, Co recovery from spent LIBs reached 94.1 %. The process was finalized by the precipitation with either Na2CO3 or (NH4)2CO3, and the final calcination to obtain Co3O4 [229].
PolyEG is a non-ionic polymer made up of repeating EG units that acts as HBD through its oxygen atoms, which can engage in hydrogen bonding in solution and does not impact DES acidity. In PTSA:ChCl DES, both compounds are acidic, which significantly helps in the leaching of LCO. This DES can leach LCO cathode in 15 min, while PTSA:EG DES needs 24 h to leach the cathode on similar temperatures. Suriyanarayanan et al. [231] have demonstrated a novel and efficient method for Co extraction from spent LCO-based LIBs using a DES made of N-methyl urea and acetamide. Previously, cathode was treated with N-Methyl-2-Pyrrolidone to separate aluminum foil from active cathode material. The product, after leaching in DES, was dissolved in 1 % CH3COOH and 0.1 M KNO3. Co was electro-deposited, dried, and calcinated before adding LiOH for fabrication of new LIBs. Other DES combinations, parameters, and leaching efficiencies are shown in Table 12 below.
Compared to LCO cathode material, NMC cathode material contains other types of transition metals Ni and Mn, which makes leaching and separation more difficult. ChCl is an extremely popular hydrogen donor in DES for NMC leaching. Combined with organic acids, leaching temperature decrease significantly [239], compared with ChCl:EG combination [240] (Table 13). Mixing DES with DMSO did not improve the process leaching time.
| DES | Temperature (°C) | Time | Solid/Liquid ratio | % recovered | Ref. |
|---|---|---|---|---|---|
| ChCl:LAC (2:1) | 50 | 1 h | 1:25 | Li 96, Mn 96, Co 96, Ni 96 | [239] |
| ChCl:LAC:CIT (3.3:5.3:1.3) | 55 | 3 h | 1:50 | Co 99 | [241] |
| ChCl:OXA + DMSO | 120 | 10 h | 1:20 | Co 95, Ni 99, Mn 95 | [242] |
| ChCl:OXA | 110 | 20 min | 1:50 | Co 100, Mn 100, Ni 100 | [223] |
| ChCl:LAC (1:2) + H2O2 | 45 | 6 h | 1:20 | Li 86.7, Co 88.5, Ni 84.5 | [243] |
| ChCl:PPA (1:2) + H2O2 | 45 | 6 h | 1:20 | Li 100, Co 98.7 | [243] |
| ChCl:PTSA·H2O (1:1) | 90 | 2 h | 1:60 | Li 97.96, Ni 99.46, Co 100, Mn 100 | [244] |
| ChCl:AA | 120 | 12 h | 1:50 | Li 100, Mn 99.2, Co 100, Ni 100 | [245] |
Xu et al. [246] used microwave assisted ChCl:OXA DES for LMO cathode leaching. Best conditions were 15 min leaching at 75 °C at solid/liquid ratio 1:60. Under these conditions, 96 % of Li and Mn is leached.
Three-component DES systems are shown in Table 14. ChCl DES requires higher temperatures and longer leaching time compared to systems without ChCl [247], as a hydrogen donor. DES without organic acid, such as ChCl:urea:EG, has the lowest leaching efficiencies for transition metals [248].
| DES | Temperature (°C) | Time | Solid/Liquid ratio | % recovered | Ref. |
|---|---|---|---|---|---|
| ChCl:urea:EG (1:2:1) | 100 | 72 h | 1:50 | Li 97, Co 41, Ni 40, Mn 34 | [248] |
| ChCl:EG:PTSA | 100 | 72 h | 1:100 | Li 97, Co 97, Ni 97, Mn 97 | [249] |
| TOPO:decA:HCl | 100 | 4 h | 1:23 | Co 100, Mn 100, Ni 90 | [148] |
| DMT:OXA:H2O | 60 | 15 min | 1:40 | Li 99, Co 0.2, Ni 0.1, Mn 8.3 | [225] |
| ChCl:EG:TAC | 120 | 10 min | 1:200 | Ni 98.8, Co 100, Mn 100 | [250] |
| ChCl:CIT:H2O | 110 | 4 h | 1:50 | Li 99.88, Ni 99.90, Co 99.77, Mn 100 | [236] |
| ChCl:LAC:TCCA | 60 | 2 h | 1:3.8 | Co 47.9, Ni 57.2, Li 56.2, Mn 59.7 | [251] |
| ChCl:MAL:glycine + H2O2 | 60 | 2 h | 1:3.8 | Co 60.1, Ni 66.1, Li 66.8, Mn 65.0 | |
| ChCl:SAC:TCCA | 60 | 2 h | 1:3.8 | Co 53.5, Ni 44.3, Li 73.4, Mn 73.4 | |
| ChCl:GUC:TCCA | 60 | 2 h | 1:3.8 | Co 51.4, Ni 49.6, Li 59.9, Mn 65.1 | |
| ChCl:CIT:PHM (1:1:1) | 60 | 2 h | 1:3.8 | Li 87.7, Mn 73.7 | |
| ChCl:CIT:H2O2(1:2:1) | 60 | 2 h | 1:3.8 | Co 51.5, Ni 59.3, Li 50.8, Mn 61.8 |
EG is another common component for DES. Acidic components such as OXA, Sulfosalicylic acid dihydrate, and LAC improve leaching conditions compared to ChCl:EG DES, as shown in Table 15. Schiavi [240] and Wang [252] proposed very similar processes for selective Co extraction from NMC cathode. In ref. [240] differences in solubility of Co and Ni were exploited by increasing the DES temperature. The lower solubility on temperatures lower than 180 ℃, found for Ni as compared to the other metals contained in the waste material may be explained by the formation of complexes with Cl− and EG. Unlike the other metals (Me), which can form stable [MeClj]n− species, Ni is the only metal coordinated by glycol molecules, thus forming [Ni(EG)3]2+ complexes [240]. EG is not expected to be a good ligand in its protonated form, and thus the formation of [Ni(EG)3]2+ may be only promoted at the temperature above 180 °C [252].
| DES |
Temperature (°C) |
Time |
Solid/Liquid ratio |
% recovered | Ref. |
|---|---|---|---|---|---|
| ChCl:EG (1:2) | 160 | 24 h | 1:50 | Co 90, Ni 10 | [240] |
| ChCl:EG (1:2) | 180 | 24 h | 1:80 | Li 91.63, Co 92.52, Ni 94.92, Mn 95.47 | [252] |
| ChCl:EG (thermally treated) | 180 | 4 h | 1:50 | Co 100, Ni 93, Mn 94 | [253] |
| BHC:EG (1:5) | 140 | 10 min | 1:40 | Li 99, Mn 99, Co 99, Ni 99 | [254] |
| BHC:EG (6:1) | 140 | 20 min | 1:25 | Ni 99.7, Co 99.6, Mn 99.1, Li 99.5 | [255] |
| EG:OXA·2 H2O (1:2) | 90 | 12 h | 1:62.5 |
Li 94.4, Co 1.2, Ni 1.2 Mn 1.2 |
[256] |
| EG:sulfosalicylic acid dihydrate·2 H2O (12:1) | 110 | 6 h | 1:25 | Li 100, Co 94.8, Ni 99.1, Mn 100 | [257] |
| EG:MAC (3:1) | 90 | 6 h | 1:33 | Li 95.63, Ni 84.63, Co 92.74, Mn 91.31 | [258] |
| EG:CIT (2.5:1) | 95 | 10 h | 1:67 | Li 99.1, Co 96.2, Ni 97.6, Mn 98.3 | [259] |
| EG:TAC (5:1) | 120 | 12 h | 1:50 | Li 98.86, Ni 0, Mn 0, Co 0 | [238] |
| EG:DMPT (3:1) | 100 | 1 h | 1:50 | Li 99.59, Ni 99.28, Co 99.04, Mn 99.45 | [260] |
Wang et al. [252] after successful leaching performed coextraction of Ni, Co, Mn and Li using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (P507) in sulfonated kerosene as the diluent. H2SO4 was added as stripping agent to recover Ni, Co and Mn from P507 solution. The post-process economic analysis revealed significant benefits of NMC regeneration through recycled materials, notably in cost reduction. Synthesizing NMC cathodes from raw materials incurred a cost of $189.49/kg, whereas using recycled materials brought it down to $144.77/kg, saving $44.72/kg in cathode production costs.
Other popular types of DES used for cathode recycling contain organic acids as HBDs and different HBAs (Table 16).
| DES | Temperature (°C) | Time |
Solid/Liquid ratio |
% recovered | Ref. |
|---|---|---|---|---|---|
| Benzethonium chloride:LAC + H2O2 | 45 | 6 h | 1:20 | Co 89.2 | [243] |
| BHC:LAC | 110 | 18 min | 1:50 | Co 99.7, Ni 99.3, Mn 99.0, Li 99.9 | [237] |
| BHC:CIT | 80 | 30 min | 1:50 | Mn 99.2, Ni 99.1, Li 99.8, Co 99.8 | [261] |
| BHC:FA | 140 | 6 h | 1:50 | Li 98.03, Ni 96.01, Co 94.19, Mn 92.35 | [262] |
| TEAC:AAC (1:1) | 80 | 2 min | 1:50 | Ni 99.6, Co 99.4, Mn 99.3, Li 99.1 | [263] |
| TBAC:MCA (1:3) | 100 | 7 h | 1:15 | Li 100, Co 100, Ni 100, Mn 100 | [264] |
| ChCl:glucose | 100 | 24 h | 1:10 | Li 93.7, Co 94.2, Mn 97.6, Ni 82.4 | [265] |
| GH:FA | 140 | 40 min | 1:50 | Li 99.79, Ni 98.86, Co 99.51, Mn 96.12 | [266] |
Kozhevnikova et al. [144] applied hydrophobic DES for metal separation from three LIBs after HCl leaching. Two systems were chosen for metal separation [A336][Cl]:menthol (1:1) and D2EHPA:menthol (1:1). The process was carried out in multiple steps at different conditions for each metal. The DES:water phase was always 1:1. Each metal extraction step was followed by washing step for DES recovery, and further metal separation. All conditions are shown in Table 17.
| DES | Number of extraction steps | Number of washing steps | % recovered | |
|---|---|---|---|---|
| LIB 1 | [A336][Cl]:menthol (1:1) + 0.5 M HCl | 5 | 6 with H2O | Cu 98.47 |
| [A336][Cl]:menthol (1:1) + 4 M HCl | 9 | 1 with H2O | Co 99.00 | |
| D2EHPA:menthol (1:1), pH = 2.5 | 2 | 1 with 1 M HCl | Al 99.00 | |
| LIB 2 | [A336][Cl]:menthol (1:1) + 0.45 M HCl | 4 | 1 with H2O | Fe 99.47 |
| [A336][Cl]:menthol (1:1) + 4 M HCl | 7 | 6 with H2O | Co 98.99 | |
| D2EHPA:menthol (1:1), pH = 2.5 | 6 | 1 with 1 M HCl | Al 99.54 | |
| LIB 3 | D2EHPA:menthol (1:1), pH = 2.5 | 4 | - | Fe 99.75, Al 99.98 |
Metals can undergo further separation through various techniques, enhancing the purity of the resulting materials. Two prominent methods for achieving this separation are precipitation using OXA [221], [227], [247], [257], [267], and high-temperature calcination to produce metal oxides [227], [242], [268].
6.4. Supported Liquid Membranes
In liquid-liquid separations, the hydrophobic phase (IL or DES) can be immobilized onto a supporting membrane, which separates a feed aqueous phase containing the metals to be separated into the IL/DES and a receiving phase where the separated metals are collected [269], [270], [271], [272]. One notable advantage of supported liquid membranes (SLMs) is the reduced amount of solvent employed with respect to liquid-liquid biphasic systems, which could be useful for the industrialization of separations involving ILs, given their relatively high cost. Moreover, extraction and stripping occur in a single step (Fig. 9). On the other hand, a drawback is the limited life due to the leakage of IL. To extend the lifetime of the membrane, polymer inclusion membranes (PIMs) have been proposed as an alternative [273], [274], [275], [276].
 Fig. 9
Fig. 9A recent work [276] studied the application of PIMs ([A336][Cl] and [P66614][Cl]) in the separation and recovery of Co and Li from LIBs, achieving good recovery performances with a long-term stability.
Co extraction using [P66614][Cl] in a SLM demonstrated up to 86 % extraction with high selectivity over Ni from a synthetic solution [277]. While stripping with deionized water is possible, reusability is hindered by IL leaching. While Zante et al. [278] assessed the potential for selectively recovering Li from aqueous solutions containing Na, Co, and Ni ions using a SLM created by a PVDF membrane support with a blend of [C4mim][Tf2N] and TBP as the carrier. Notably, their stability experiments revealed that the leakage of IL into the aqueous phase could be minimized by introducing salt into either the feed or the stripping phase.
DES-based SLMs exhibit distinct advantages, such as lower solvent loss and improved efficiency in liquid separation without loading limitations [279]. The chemical potential gradient in DES-based SLMs facilitates mass transfer on both sides, ensuring durability as the feed solute does not chemically react with the DES carrier during passive diffusion.
However, the fabrication process depends on parameters like DES type, composition, membrane support thickness, wettability, and additives [280], [281], [282]. Achieving long-term durability necessitates a reliable match between DES and its membrane support, an aspect requiring considerable investigation effort.
However, several challenges hinder their large-scale application of SILMs [272]. Current preparation methods are intrinsically inefficient as they basically rely on a trial-and-error approach. More systematic screenings involving molecular simulations [283] and machine learning techniques [284] could help to improve this aspect. Balancing permeability, selectivity and useful membrane lifetime remains a significant challenge. Innovations in interface regulation and mass transfer optimization through a more rational preparation would help in this direction.
6.5. Reusability of ILs and DES
In the quest for the reuse of metal-free ILs and DESs, specific procedures are indispensable, frequently involving multiple washing steps with strip solutions to eliminate any residual metals and solution [204], [285], [286], [287]. The recyclability of ILs and DESs hinges largely on their physico-chemical properties. Hydrophilic ILs, which encompass cations like ammonium and phosphonium along with anions such as [Cl]–, tend to exhibit reduced recyclability due to the loss of anion during water washing. For instance, [A336][Cl] and [P66614][Cl] [288] displayed an 8 % decrease in extraction efficiency ( i.e., the ratio of extracted metal to the total amount in the feed) even after a single washing cycle.
On the contrary, hydrophobic ILs like [P66614][TMPP] and [P66614][Tf2N], characterized by hydrophobic anions [289], demonstrate superior recyclability, retaining their extractability across multiple extraction-stripping cycles. This disparity in recyclability has posed significant challenges when scaling up IL-assisted solvent extraction processes, especially for chloro-complexes that involve ion-exchange reactions. The loss of IL molecules diminishes the availability of extractants, necessitating additional treatments to recover the dissolved IL molecules in aqueous solutions. Recognizing the importance of the recovery and reuse of ILs and DESs is fundamental for the successful commercialization of applicable technologies, as it plays a pivotal role in efficient resource utilization and environmental preservation [290], [291], [292]. The approach to recycling these compounds typically depends on their hydrophobic or hydrophilic nature. Consequently, researchers have been actively exploring the recycling of spent LIBs using ILs and DESs.
6.6. Transition to industrial applications
Despite the academic research on the application of ILs/DESs in the recovery of metals from LIBs for more than a decade (Fig. 2), and the promising performances achieved in selective extraction processes on the lab scale, currently, there are no commercial processes for metal recovery from LIBs employing ILs or DESs. More generally, it should be noted that also the full industrialization based on IL/DES-based processes for other metal extractions has not been reached yet. The only IL-based separation process known is related to rare earth recycling from permanent magnets [293], which is currently in an advanced demonstration stage (Ionic Technologies, former Seren Technologies [294]).
As for pilot scale plants, while some examples on LIBs recycling based on other technologies, such as multi-step solvent separation [295], smelting [296], or selective precipitation [171], are available, similar examples based on ILs/DESs are not reported to date. On the other hand several lab-scale plants relevant to LIBs recycling employing continuous bench-scale processes have been reported for Co/Ni separation [297], [298], also employing microfluidic reactors [299].
However, the interest in moving towards industrial applications exists, as the number of patents related to metal recycling from LIBs has constantly increasing in the last decade (a search on Google Patents reports 89 results from 20101).
One of the main barriers for the industrialization of processes employing ILs and DESs for separations is their cost, which is much higher than VOCs (Table 7) [301]. The prices of ILs and DES are strongly dependent on the specific solvent, and estimations based on chemicals catalogues may not be reliable as they are often referred to small quantities of research-grade products. Specific studies on economic feasibility of metals recycling from LIBs have not been published. However, some information can be obtained from other works on applications of ILs/DESs, where the cost of these materials has been estimated on an industrial scale.
A first study on techno-economic aspects of an industrial gas purification process involving ILs estimated a cost of IL ranging from 2.5 to 50 USD kg–1, around 2–50 times higher than that of common VOCs employed in industry [302], [303]. Other analyses of applications of ILs and DESs for gas treatment estimated an average price of 6000 USD/t for some imidazolium-based ILs [304].
The first route to reduce the production cost is to optimize the synthetic procedures to prepare ILs. In this respect, several examples of studies where optimized syntheses are available [305], [306], [307]. Also, replacing more expensive ILs with cheaper ones can also contribute to reducing prices [308]. Besides improvements in chemical and process aspects, it should be taken into account that the ILs market is expected to grow, and the prices should decrease due to economies of scale as larger volumes of ILs are produced [309]. It has been reported that an increase of production from 1 kg to 5 tons for two imidazolium ILs can generate a decrease of the cost per kg of more than a factor of 3 [301].
DES are generally much less expensive than ILs as they are produced by simply mixing separate chemicals, which in some cases have an individual low cost (e.g., urea).
In a simulated process [310], the ChCl:urea (1:2) DES was considered with an estimated price of around 600 USD/t, 10-fold less than the IL employed in the same process [304]. A study [311] on the use of ChCl:urea (1:2) in a bio-refinery estimated 200 and 90 USD/t for the two components of the DES, respectively. A techno-economic analysis on biogas upgrading showed that DES-based process could be competitive in cost with current technology [312]. Also, natural DESs (NADEs), which have been proposed as solvents for bio-refineries, were considered suitable for an industrial process [313]. Therefore, the results of these studies demonstrate the economic feasibility and competitiveness of industrial processes based on DESs.
Additional factors which can help to mitigate the price issue are the use of low volumes of ILs and DESs (for example by coupling them with membrane supports), extending the operating lifetime and their recycling.
7. Conclusions and Outlook
The increasing pressure on the supply chain for CRMs used to manufacture LIBs and the environmental risks related both to primary materials supply and waste disposal highlight the importance of recycling. Due to this increasing demand, there is an urgent need to develop an environmentally friendly and cost-effective recycling technology to address current challenges.
ILs and DESs have shown promising potential in separation of metals in hydrometallurgical recycling EoL LIBs, as evidenced by numerous research works. However, their application is still limited by economic and chemical limitations (Fig. 10). Nevertheless, their low volatility reduces the risk of emissions, enhancing safety during handling compared to VOCs. Furthermore, the potential for multiple cycles of reuse makes ILs environmentally sustainable, contributing to cost reduction. Their chemical structure is highly adaptable, facilitating customization for specific metal recovery applications. Phosphonium-based ILs shows promising potential for extracting Co from various types of cathodes in LIBs, with recovery percentages varying based on the specific IL, cathode type, A:O ratio, and extraction conditions. Furthermore, the synergistic utilization of ILs with extractants holds significant promise for Li extraction, with the recovery percentages being subject to the operational conditions.
 Fig. 10
Fig. 10On the other hand, DESs, often considered more “environmentally friendly” than ILs as their components are often biodegradable and of low cost, have also shown promising results. Like ILs, DESs can be designed for selective metal extraction, providing advantages in separating different metals present in spent LIBs. Specifically, formulations incorporating acidic components such as GH and OXA show a significant reduction in the leaching temperature of LCO cathode. For NMC cathode materials, formulations with ChCl have demonstrated effectiveness, particularly when combined with LAC. Furthermore, metals can undergo further separation utilizing various techniques such as precipitation and calcination. Nevertheless, the process of metal recovery using DESs may involve energy-intensive steps, due to the need to operate at moderately high temperature to reduce viscosity, impacting overall energy efficiency.
SLMs, where the hydrophobic IL/DES is immobilized onto a membrane, offer reduced solvent usage and single-step extraction and stripping. However, concerns arise over limited membrane lifespan due to leakage. To address this, PIMs could serve as an alternative, as suggested for Co and Li separation and recovery, showing promising performance and long-term stability.
Thus far, only a limited selection of ILs and DESs have been employed for the metal recovery from spent LIBs. The future holds substantial potential to broaden the variety of components utilized and consequently extend the applications of these solvents.
CRediT authorship contribution statement
Marilena Tolazzi: Writing – review & editing. Anđela Kovačević: Writing – review & editing, Writing – original draft. Andrea Melchior: Writing – review & editing, Writing – original draft, Supervision, Resources, Funding acquisition, Conceptualization. Martina Sanadar: Writing – review & editing, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the University of Udine, in the framework of the Strategic Plan 2022–25– Interdepartmental Research Project ESPeRT. AM&MS acknowledge the University of Udine for Research financed with European Union funds – NextGenerationEU—MSCA grants D.M. 737/2021-CUPG25F21003390007. AM&AK acknowledge the PhD programme on Green topics (Action IV.5, DM 1061/2021, cycle XXXVII) carried out with co-financing from the European Union - ESF REACT-EU, PON Research and Innovation 2014–2020, UniUD. MT&MS acknowledges the Italian Ministry for University and research for funding through the PRIN2022 call with the project “Wastezilla” (2022HYH95P).
Data availability
No data was used for the research described in the article.
- 1
-
Using the string in ref. [300]
© 2024 The Author(s). Published by Elsevier Ltd.